The Atomic Elements & Isotopes
Atoms
We have all heard about atoms, and directly experience the elements they form. Iron, copper, gold and silver are everyday stuff. We have also heard that atoms are made from neutrons protons and electrons. The neutrons and protons are part of a small central positively charged nucleus, while the negatively charged electrons exist in “orbitals” within a much larger volume. It is commonly said that most of the atom’s volume is empty space.
The atomic elements are the stuff the cosmos makes after it has made the protons, electrons, and the neucleons. There are 92 naturally occurring elements with an average distribution in nature that can be estimated based on our astronomical observations.
The atomic concept dates back thousands of years in human history. There are many good sources readily available that describe this history in detail. Atomic history is not the purpose of this work.
By the 1950’s, the basic model of the atom was visualized as consisting of a nucleus – a small spherical body consisting of a number of densely packed positively charged protons and neutral neutrons – surrounded by negatively charged electrons (equal to the proton number in a neutral atom) that occupied the much larger volume of the overall atom.
Most of the atom’s mass (more than 99.8%) is contained within the volume of the nucleus. The electrons carried only a small fraction of the atom’s total mass. Once an estimate of the physical size of the nucleus was made, its density (was found to be enormous, more than one hundred trillion times greater than water. The atomic volume on the other hand was practically empty, containing just the small mass of the dynamic electrons.
It seemed clear that the negatively charged electrons were bound to the positively charged nucleus because of the attraction between unlike electrical charges and repelled by each other because of the repulsion between like electrical charges. This is the well known Coulomb force. By the 1930’s an electron shell model (quantum electrodynamics) successfully described basic atomic architecture and its interaction with radiation.
Basic Atomic Concepts
- An atom is a small dense positively charged nucleus surrounded by number of negatively charged electrons that occupy a volume that is more than 4 orders of magnitude larger than the nucleus. For example the diameter of a helium nucleus is 1.68×10-15 m, 18,452 times smaller than its atomic diameter of 62×10-12 m.
- Electrons occupy ordered shells that are well described by Current Science. The Neu Theory conceptual addition to the current model is, that the electric hollows [10] are identified as the physical place in the atomic volume that mediate the formation and absorption of photons.
- When the number of negatively charged electrons equals the number of positive nuclear charges the atom becomes electrically neutral.
- When the number of electrons is larger than the number of nuclear charges, the atom becomes a negatively (-) charged ion.
- When the number of electrons is smaller than the number of nuclear charges the atom becomes a positively (+) charged ion.
- When the nucleus of an atom has no electrons to balance its positive charge, it is defined as being fully ionized. The cosmic ray shower is made from fully ionized nuclides.
- Atomic photons. These are photons created in electric hollows by electrons interacting with nuclear charge. The energy of an atomic photon is equal to the reduction in potential energy of the electric field. Atomic photons vary in physical size (wavelength), with even the smallest and most energetic photons (x-rays), being thousands of times larger in diameter than the atoms that formed them.
- Nuclear photons. These are photons (gamma rays) created by nuclear events. Gamma rays vary in physical size (wavelength) with even the smallest and most energetic gamma rays being thousands of times larger in diameter than the nuclides that formed them.
The Electron Parking Structure
Using existing concepts, atomic structure can be modeled as a parking garage building for dynamic electrons. Each atomic garage is unique, although built from a standard set of specifications. In all atoms, the underlying topological design remains constant. See Figure 4.1 – Uranium Electron Parking Levels.
There are seven (7) main parking levels named in order with the letters K, L, M, N, O, P, and Q. At each main parking level there are one to four (1- 4) parking sub-shells named s, p, d, and f. Levels and sub-shells only exist if they are occupied by an electron.
Neu Theory adds the concept of the electric hollow [10] in between each main level of electrons. The electric hollows are named by adding the italicized lower case of the main shell letter to the fundamental form number. Thus [10k] is the electric hollow shell between the K electron shell and the charge shield of the nuclide, [10l] is the electric hollow between the L electron shell and the K electron shell.
The electrons can be visualized as paying a parking fee, from the potential energy of their electric field, as they dynamically park at a specific speed, in some available place in the spherical positive electric field garage. The cost of the parking ticket is paid with the energy of the released photon.
The electric hollows below the level where the electron is parked, determines the cost of each ticket, issues the photon, and adjusts the residual electric field appropriately. The photon ticket can be redeemed by an electron, allowing it to park in a higher shell at a slower speed, or leave the atomic parking structure entirely (the photo-electric effect).
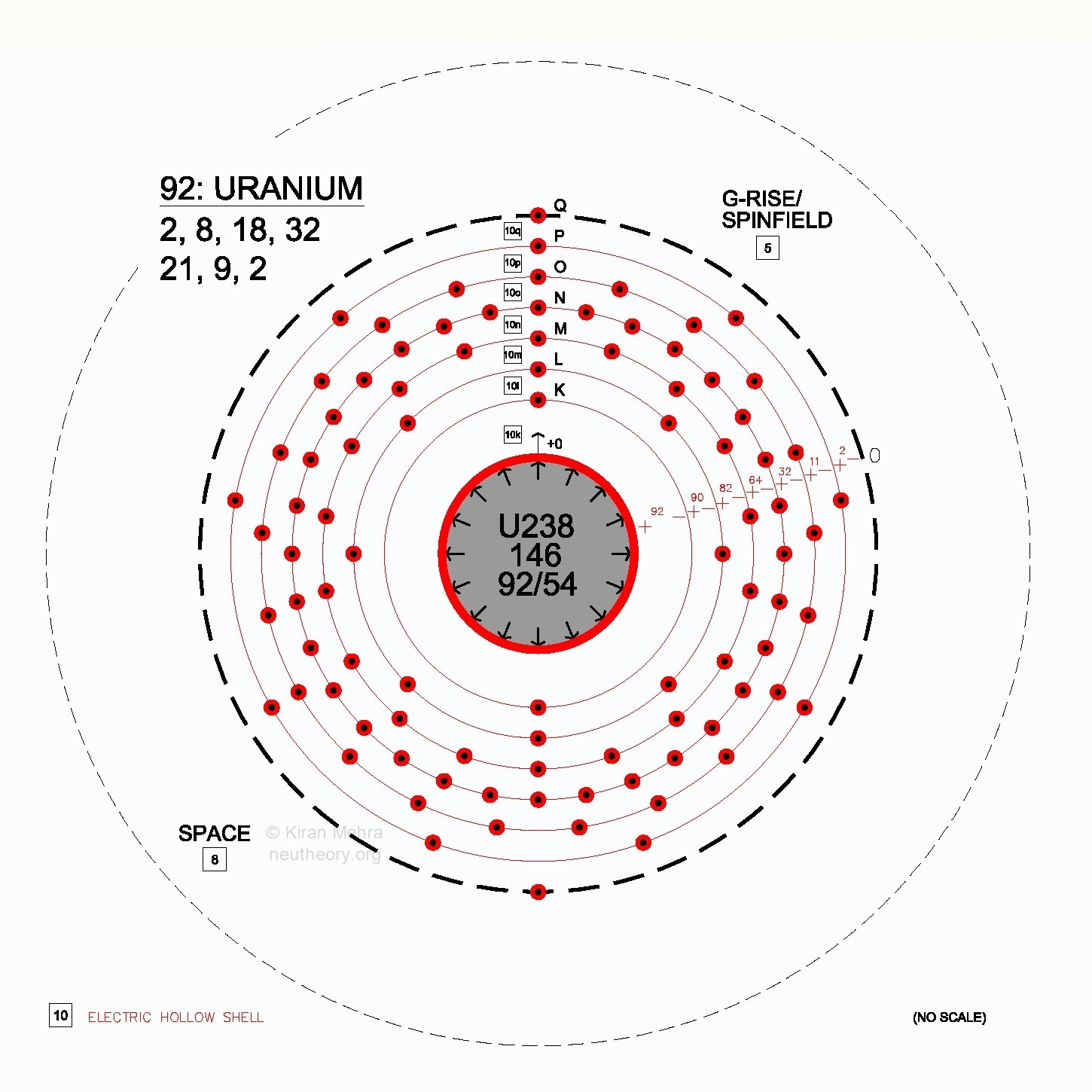 Figure 4.1 – Uranium Electron Parking Levels
Figure 4.1 – Uranium Electron Parking Levels
Each parking sub-shell has stalls for (s=1, p=3, d=5, f=7) electron pairs. There are a total of 118 parking stalls available in the standardized atomic parking structure design corresponding to the Periodic Table of the Elements. See Figure 4.1 for the parking structure of *Uranium-238 with 92 electrons, the last naturally found isotope.
Each atomic element builds its own parking structure by establishing the number of main levels, and then filling the available parking stalls in each sub-shell in that level with electrons in a specific order. Not all available stalls in a sub-shell are necessarily filled before the dynamic electrons start occupying parking stalls in the next higher sub-shell or the next higher main level.
As an example, the first use of parking level 7 is Francium with its 87 electrons. The 87th electron parks itself in one of the two stalls in the 7s sub-shell instead of starting the 5f or 6d sub-shells which are completely open. The next (88th) electron with the element Radium occupies the second of the two 7s stalls. The 7s sub-shell of all atoms after Radium is always filled. *
* “The Elements” by Theodore Grey
Main Electron Parking Levels
- (K) 2 spaces – Begins with Hydrogen (1 electron), (1s sub-shell filled with Helium (2 electrons))
- (L) 8 spaces – Begins with Lithium (3 electrons), (2s, 2p sub-shells filled with Neon (10 electrons))
- (M) 18 spaces – Begins with Sodium (11 electrons), (3s, 3p, 3d sub-shells filled with Copper (29 electrons))
- (N) 32 spaces – Begins with Potassium (19 electrons), (4s, 4p, 4d, 4f sub-shells filled with Ytterbium (39 electrons))
- (O) 32 spaces – Begins with Rubidium (37 electrons), (5f sub-shell never filled, highest stable atom is Bismuth (83 electrons))
- (P) 18 spaces – Begins with Cesium (55 electrons), (6d sub-shell never filled, highest stable atom is Bismuth (83 electrons))
- (Q) 2 spaces – Begins with Francium (87 electrons), (7s sub-shell always filled, 7p shell occupancy not observed; no stable atoms)
Atomic Size
Atoms have a finite size that can be defined by 3 quantities; the nuclear charge radius, the atomic radius, and in Neu Theory the spinfield radius:
- charge radius, a reasonably precise quantity, represents the size of the atomic nucleus. Neu Theory uses the charge radii values empirically determined and published in September 1999 by the International Nuclear Data Committee of the International Atomic Energy Agency. The measured charge radius of the proton (0.84184×10-15m) is the natural standard for physical size in nature. Of the naturally occurring atoms the smallest charge radius is with helium-4 ∼1.6758×10-15m, the largest charge radius is with uranium-238 ∼5.8473×10-15m.
- atomic radius, a fuzzy boundary, represents the distance from the atomic nucleus to the outermost electron orbital. All isotopes of a particular atom have similar atomic size. The smallest is helium ∼31×10-12 m, the largest is cesium ∼298×10-12 m, almost 10 times larger.
- spinfield radius, an approximate minimum boundary, represents the size of the volume surrounding the atomic radius which cannot be occupied by another atom (packing or Van der Waals radius). It is the spinfield radius of atoms that determines nonbonded crystalline structure.
